Stainless steels, stainless steel corrosion, and proper chemical treatment of Inox steel, i.e. degreasing, pickling, and passivation
Why does stainless steel rust, in what situations and how to protect stainless steel against corrosion? The answers to these questions are asked by people from the stainless steel processing industry, but also by users of stainless steel products. In the guideline below, we will tell you about the correct processing of stainless steel.
First of all... what are stainless steels?
Stainless steel contains a maximum of 1.2% carbon and a minimum of 10.5% chromium (according to the European standard EN-10088). Steels of this group may also contain other alloying elements such as nickel, molybdenum, niobium, nitrogen, copper, titanium, magnesium, or sulfur.
Depending on the composition of the alloy, the following types are distinguished:
- Austenitic, e.g., 1.4301, 1.4401
- Ferritic, e.g., 1.4016, 1.4521
- Martensitic, e.g., 1.4031
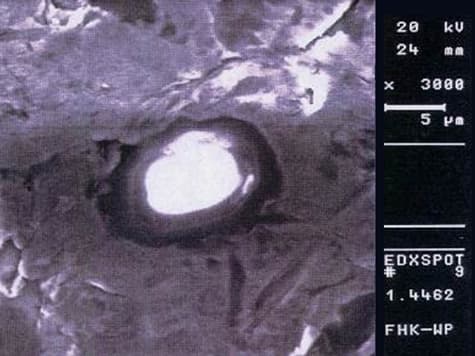
- Duplex, e.g., 1.4362, 1.4462
The expected corrosion resistance is a key criterion for selecting steel. The use of stainless steel is increasingly common for visible parts such as facades, windows, doors, and kitchens, where aesthetic appearance is particularly important. However, these fundamentally positive properties can lead to disappointment if steel processing processes are improperly selected and poorly executed.
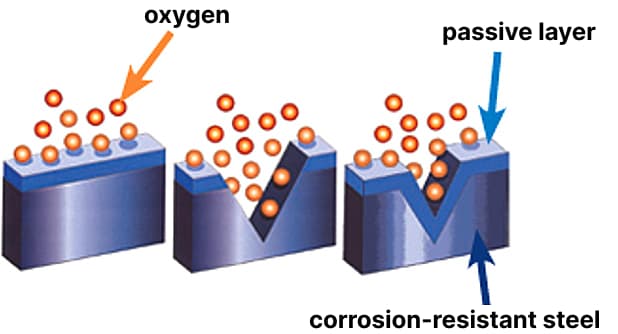
An intact and fully formed passive layer of steel is essential to ensure corrosion resistance. This can only be achieved through optimal treatment of the surfaces of products made from special steel in the final stage of processing.
The corrosion resistance of stainless steels is primarily a function of the chemical composition of the alloy, the structure, and the quality of the protective layer of chromium oxide formed on the surface. The passive layer - a chromium oxide layer no thicker than 2-4 nm - forms spontaneously when the chromium content in the alloy is higher than 12%. It prevents direct contact of the steel with the surrounding environment and thus protects it from corrosion. In addition to chromium content, the spontaneous formation of the passive layer requires meeting the following criteria: a clean surface and a sufficient amount of oxygen for the reaction. If any of these conditions are not met, this layer does not form spontaneously, and the corrosion resistance is significantly reduced.
The protective layer is destroyed as a result of processing stainless steel through processes such as drilling, turning, milling, or bending. Normally, the protective layer is immediately regenerated provided that the surface remains free from contaminants such as inclusions of foreign metals, for example, caused by processing carbon steel material on the same machine, or contaminants due to traces of footwear, sweat from hands, and dust. Damage to the surface of the passive metal layer leads to progressive rusting. Welding also destroys it. The oxides formed as a result of welding are the cause of the electrochemical corrosion process. This can only be prevented by carefully removing the oxide layer after welding.
Corrosion resistance not only depends on the formation of the chromium oxide layer but also on its stability in the surrounding environment. When the surface of corrosion-resistant steel is penetrated due to external factors, it can lead to significant damage from various types of corrosion in a relatively short period.
Why does stainless steel also corrode?
Corrosion of most metals is an inevitable, spontaneous process. Most metals corrode if there are factors in the surrounding environment that promote this reaction. From a thermodynamic point of view, the state of the metal is unstable and metals tend to move to an energetically more favourable state, for example to an oxidised state like metal oxides.
What types of corrosion occur?
Surface
Surface corrosion is characterized by uniform surface degradation through corrosion. When the protective layer of stainless steel is destroyed by the corrosive environment, the entire surface rusts. This type of corrosion is typical for unprotected carbon steels. It is less common in stainless steels used in construction because the corrosive conditions are usually not aggressive enough to cause it (typically occurs in acidic environments).
Pitting Corrosion
Pitting corrosion can occur in various materials such as aluminum and titanium, non-alloy and low-alloy steels, as well as high-alloy chromium-nickel steels. This type of corrosion describes localized surface attack, resulting in needle-shaped holes that expand beneath the surface.
In practice, this phenomenon is often caused by the presence of chlorine, for example, from the use of technical products containing chlorine (e.g., cleaning agents, anti-scaling agents, disinfectants, chemical preparations in wastewater treatment plants).
Chlorine has a catalytic effect on pitting corrosion, which is initiated by the interaction of chlorine ions with the passive layer. As a result, the protective layer is penetrated and damaged. Rust spots form at these locations, contributing to its further progression. The subsurface corrosion spot becomes the anode and is characterized by a high rate of metal degradation. The remaining passive layer acts as the cathode, where oxygen is reduced.
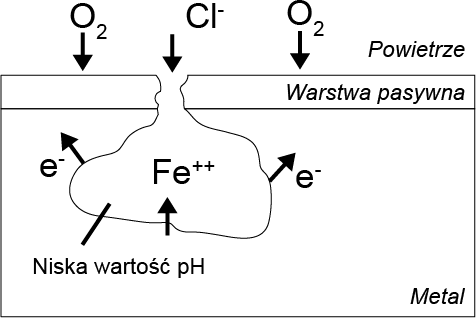
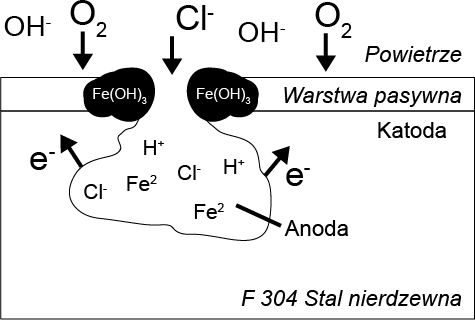
When the exchange of substances is hindered, the internal cavity lacks the oxygen necessary for the reformation of the passive layer. Through the hydrolysis of metal chlorides, the pH value in the cavity decreases, indicating that pitting corrosion is accelerated until the material is locally destroyed in a very short period of time.
Crevice Corrosion
This is an electrochemical process, similar to pitting corrosion. The only difference lies in the initial conditions. Adhesive and capillary forces act in the crevices, locally impeding fluid exchange. These areas exhibit low atmospheric oxygen concentration, which inhibits spontaneous repassivation, consequently leading to metal erosion.

When comparing pitting corrosion and crevice corrosion, it can be observed that crevice corrosion occurs at significantly lower exposure to conducive factors. Therefore, it is important not to allow the formation of conditions favorable to the occurrence of crevice corrosion during the design and execution stages.
Intergranular corrosion
Does welding stainless steel affect corrosion? During heat treatment or welding of unstabilized stainless steels with additions of titanium, zirconium, or niobium, there may be a localized increase in carbon concentration. A decrease in the corrosion resistance of stainless steels may occur in the heat-affected zone of the weld.

Intergranular corrosion results from the precipitation of chromium carbides along grain boundaries, leading to a reduction in chromium concentration at these locations and a deterioration of the protective layer's stability.

Properly processed stainless steels are not susceptible to intergranular corrosion.
Stress Corrosion
Stress corrosion is caused by the formation of cracks in metals accompanied by tensile stress in a corrosive environment. Even very low levels of stress can initiate the corrosion process. They can, for example, be introduced into the component through static loads or residual stresses resulting from steel processing such as welding, grinding, or cold bending. In this type of corrosion, the progression is determined by the potential pH value, the concentration of certain ions (mainly chlorine), and the surrounding temperature.
Galvanic Corrosion
Galvanic corrosion occurs at the junction of two metals with a large difference in galvanic potentials. The more anodic metal undergoes corrosion.

To avoid galvanic corrosion, it is important to avoid connections between different metals, use insulators, or preferably use welded connections whenever possible.
Chemical processing in 3 simple steps
Degreasing, pickling process, and passivation of stainless steel surfaces to maintain corrosion resistance.
Steel processing through chemical methods used in these processes helps protect inox details from adverse effects such as oxidation and temperature-related discoloration, deposition of foreign metals and organic contaminants, without changing the surface structure. Proper selection and use of chemical agents are very important for environmentally and economically conducting stainless steel cleaning. Below is the full sequence of the chemical process for preparing stainless steel surfaces.
Degreasing of stainless steel surfaces
The most important stage of surface preparation. Proper degreasing not only ensures the removal of oil, grease, or other organic contaminants from the surface of stainless steel but also ensures effective and uniform etching of processed stainless steel. Degreasing can be acidic, neutral, or alkaline. It can be done by spraying or immersion. The choice of appropriate preparations and the dedicated degreasing method depends largely on the size and geometry of the detail, its purpose, and other requirements.
A well-degreased surface is characterized by good wetting.

The water film is uniform, does not break in streams, and streaks do not reappear.
Degreasing agents are often a multi-component mixture of specially selected components that, in addition to the acidic or alkaline base, also dissolve detergents - substances that facilitate degreasing of the processed surface.

Detergents/surfactants are designed to remove contamination from the surface and prevent its secondary deposition.
Pickling of stainless steel
Pickling of stainless steel is the only reliable method of obtaining a metallically clean surface. Pickling not only eliminates oxidation, discoloration, and foreign metals but also iron from the base material. This leads to a purer stainless steel surface on the processed detail. Pickling time and temperature are key parameters of the pickling process. The appropriately selected exposure time of the detail and temperature, depending on the pickling agent used, affect the effectiveness of the process and the final condition of the surface. The chemical pickling process, like degreasing, can be carried out by immersion or spraying. Immersion pickling seems to be the most economically viable due to the low consumption of the pickling agent in the process. However, it requires the construction of a specialized installation resistant to chemical agents and a bath control system. Pickling gel is quick to apply and widely used for large-scale details. There are also pickling pastes for local treatment surfaces, e.g. after welding.
Depending on the grade of stainless steel, pickling agents of various pickling strengths are used. It is worth emphasizing that the chemical pickling process is not only technologically important but also significant for the aesthetics and durability of stainless steel in various industrial and structural applications.

The precise methodology of pickling stainless steel is, for example, cited in ASTM A380 – practice for cleaning, removing heat tint, and passivating stainless steel parts, equipment, and systems.
Passivation of stainless steel
The result of pickling stainless steel is the spontaneous formation of a protective layer with the involvement of atmospheric oxygen. However, the purpose of chemical passivation of stainless steel is to accelerate this reaction and achieve a thicker protective layer. Commonly used passivating agents are acidic products, but there are also known non-acidic, neutral products that are more environmentally friendly. This is particularly important when finished elements are to be used directly after processing and as an additional protection for the uniform formation of the insulating layer.
Passivation is particularly useful for complex elements with overlapping welds, which are penetrated by etchants and after about two weeks, brown discolorations of stainless steel appear. The passivation process allows for better processing of these areas because it does not involve re-attacking the base material. As a result, brown spots can be avoided.
After chemical treatment, it is recommended to measure the passivation with a passivation meter, e.g., Passi Test Nitty Gritty
Passi Test is a tool for those who want to accurately assess the real quality of the passivation layer of stainless steel. It consists of a probe containing an electrochemical system and an external reading unit. The Passi Test measures the open circuit potential (and thus its corrosion resistance).
Explore our devices...See the effect before and after pickling gel.
Antox 73E SG (with dye) was used
Simultaneous cleaning, pickling, and passivation of stainless steel in the electrochemical method of cleaning stainless steel welds.
Electrochemical cleaning of weld oxides after welding stainless steel is an effective method. Compared to mechanical processing, it does not damage the passivation layer responsible for the anti-corrosive properties of stainless steel. Cleaning and passivation of steel welds occur in a single process, reducing the total processing time.
Discover our devices ...Work Safety
and Environmental Protection
Transport, storage, and use of chemical concentrates, their diluted forms, and bath solutions are subject to appropriate legal regulations.
Detailed information about the product is included in the so-called safety data sheets of the preparations.
Wastewater
Before discharge into the sewage system, all wastewater should undergo treatment in accordance with national and local legal regulations.

ANTOX® Series - specialized products for stainless steel treatment by chemical methods manufactured by Chemetall


Chemetall, as a global leader in surface treatment, has developed a complete range of products for degreasing, etching, and passivation of stainless steel.
Chemetall is a company specializing in chemical and technical processes, established in 1982 as a subsidiary of Metallgesellschaft AG in Frankfurt am Main. Since 2016, it has been a global business unit of Surface Treatment in the BASF Coatings division operating under the Chemetall brand. Chemetall has branches in 40 countries and 21 of them have their own production facilities.
Chemical surface treatment of metals is Chemetall's core competence. The company's global activities focus on developing and implementing customized technologies and systemic solutions tailored to individual needs. Chemetall's products protect metals from corrosion, facilitate forming and processing, optimally prepare parts for painting processes, and ensure excellent adhesion of coatings.
Chemetall's products are used in various industries and end markets, such as automotive, aerospace, aluminum processing, and metal forming.
Mechanical Methods, as a Last Resort
How to Mechanically Process to Minimize Corrosion Risk?
Mechanical processing of stainless steel is used when speed matters, but the effect is not always satisfactory. Brushing, grinding, or pressure processing are simple methods that are not always effective and durable. Especially when we need to process hard-to-reach areas.
Brushing
Brushing should only be used when a low level of chemical resistance is required. The processing is always done with wire brushes made of austenitic steel. This type of surface treatment is inexpensive but cannot be used in hard-to-reach areas and is not suitable for removing heavily adhered slag residues and boiler stone layers.
Brushing should only be used when a low level of chemical resistance is required. The processing is always done with wire brushes made of austenitic steel. This type of surface treatment is inexpensive but cannot be used in hard-to-reach areas and is not suitable for removing heavily adhered slag residues and boiler stone layers.
Read more ...Grinding
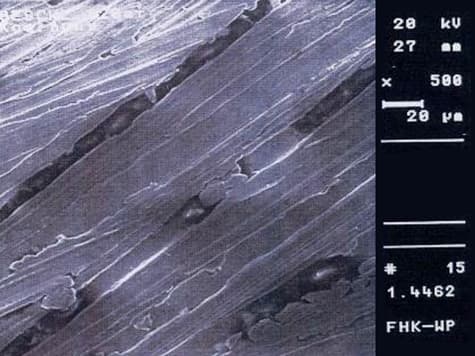
Surface processing of stainless steel by grinding is performed for various reasons. Scale and oxide layers formed by welding or thermal processing must be removed, and welding seams or surface defects must be processed. The surface quality must be improved for optical and corrosion-chemical reasons. Grinding, as a mechanical process, leads to cold surface hardening and internal stresses. This reduces resistance to pitting and crevice corrosion. The ground surface is not smooth and is characterized by many small grooves.
Blasting
This surface processing method is perfect for cleaning heavily adhered boiler stone layers and obtaining optically smooth surfaces. Abrasive jet machining with glass or ceramic balls has become the standard method for processing stainless steel. The size of the balls determines the level of surface cleanliness achieved during abrasive jet machining. Smaller balls allow for higher cleanliness than larger ones.
Abrasive jet machining also has its drawbacks, as very often abrasive material is embedded in the steel surface..
Mechanical Methods, or Speed ...
As can be seen, mechanical methods have their drawbacks and usually mean that corrosion will appear in the material sooner or later. However, if long-term resistance is not important to us, it is worth using proven tools and materials.
Discover our products ...Watch videos of the stainless steel etching process
See how spray etching looks in practice
See how immersion etching looks in practice

Contact your nearest branch.
Contact us if you want to learn more about chemical processing of stainless steel and ANTOX® products.
Copyright 2023: Chemetall - BASF, RYWAL-RHC







